Introduction Of Trimethylsilyl Trifluoromethanesulfonate:
In the realm of organic synthesis, the quest for efficient and versatile reagents is perpetual. Among the pantheon of reagents, trimethylsilyl trifluoromethanesulfonate (TMSOTf) stands out for its remarkable versatility and efficacy. In this comprehensive blog post, we embark on a journey to explore the intricacies of TMSOTf, from its synthesis to its diverse applications in organic chemistry.
Trimethylsilyl Trifluoromethanesulfonate Synthesis And Structure:
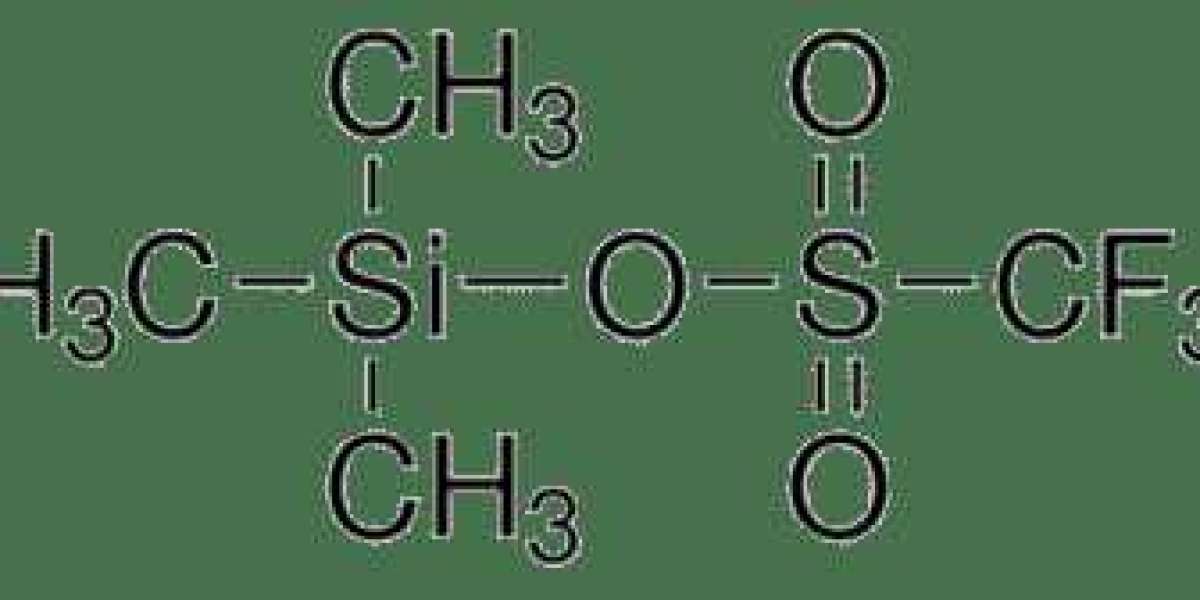
Trimethylsilyl Trifluoromethanesulfonate , with the chemical formula (CH3)3SiOTf, is synthesized through the reaction of trimethylsilyl chloride with trifluoromethanesulfonic anhydride. This process yields a colorless, volatile liquid reagent with a characteristic pungent odor. The structure of TMSOTf features a trimethylsilyl (TMS) group coordinated with a trifluoromethanesulfonate (triflate) anion, endowing it with unique reactivity and functionality.
Versatile Applications:
The versatility of TMSOTf finds expression across a myriad of synthetic transformations and methodologies:
- Acylation Reactions: TMSOTf serves as a potent acylating agent in organic synthesis, facilitating the conversion of alcohols, phenols, and amines into esters, ethers, and amides, respectively. Its Lewis acidic nature promotes the activation of nucleophiles, leading to rapid and selective acylation reactions.
- Protection and Deprotection Strategies: TMSOTf finds utility in protecting group chemistry, particularly in the silylation of hydroxyl groups to form trimethylsilyl ethers. These silyl ethers serve as temporary protecting groups, shielding reactive functionalities from unwanted reactions while allowing for selective transformations.
- Lewis Acid Catalysis: As a Lewis acid catalyst, TMSOTf participates in various organic transformations, including Friedel-Crafts acylation, Diels-Alder reactions, and rearrangement reactions. Its ability to coordinate with electron-rich substrates facilitates bond formation and rearrangement processes.
Safety Considerations:
While TMSOTf offers invaluable utility in organic synthesis, it is essential to handle it with care due to its corrosive and reactive nature. Proper safety protocols, including the use of appropriate personal protective equipment (PPE) and working in a well-ventilated environment, are imperative to ensure safe handling and minimize risks to health and safety.
Conclusion:
In conclusion, trimethylsilyl trifluoromethanesulfonate emerges as a pivotal reagent in the arsenal of synthetic chemists, empowering researchers with its versatility and efficacy. From acylation reactions to protection strategies and Lewis acid catalysis, its myriad applications underscore its indispensable role in advancing synthetic methodologies and driving innovation in organic chemistry. As research continues to unveil new synthetic strategies and applications, the legacy of TMSOTf as a transformative reagent remains unwavering, shaping the future of chemical synthesis and molecular design.