Introduction
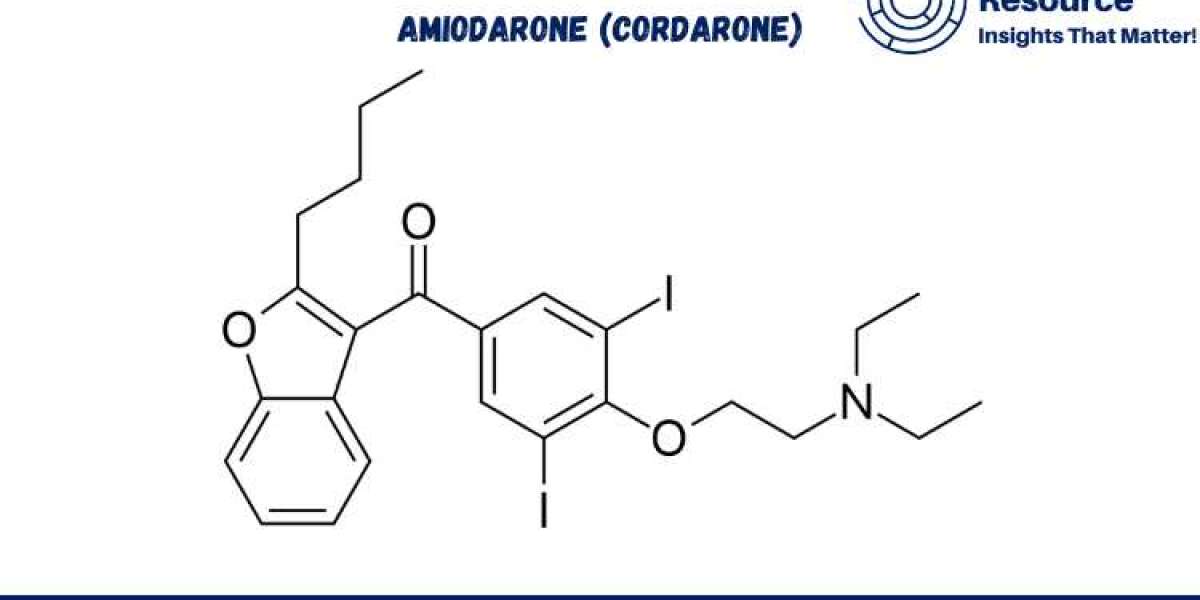
Amiodarone (Cordarone) Production Process with Cost Analysis is crucial for understanding the complexities involved in manufacturing this widely used antiarrhythmic medication. Amiodarone, sold under the brand name Cordarone, is essential in treating life-threatening ventricular arrhythmias and atrial fibrillation. The production process for amiodarone involves multiple chemical synthesis steps, precise quality control, and significant resource management. This report delves into the amiodarone production process, procurement resource assessment, market drivers, raw materials requirements, and cost analysis to provide valuable insights for manufacturers and stakeholders.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/amiodarone/request-sample
Procurement Resource Assessment: Amiodarone (Cordarone) Production Process
The production of amiodarone involves several key steps, from the sourcing of raw materials to chemical synthesis, purification, and packaging. Efficient procurement of raw materials, energy, and equipment is vital for ensuring a consistent supply of amiodarone. Key considerations for procurement resource assessment include:
Raw Material Sourcing: Amiodarone is synthesized from several complex chemical precursors, including iodinated derivatives. Sourcing high-purity starting materials is critical to achieving the desired pharmacological properties and ensuring the safety and efficacy of the final product.
Technology and Equipment: The synthesis of amiodarone involves advanced chemical reactions, including halogenation, alkylation, and cyclization. Modern production facilities must be equipped with reactors, filtration systems, and purification equipment to ensure high yield and product purity. Automation and process control systems can help optimize production efficiency and reduce manual intervention.
Energy Requirements: Amiodarone production requires significant energy inputs, particularly during chemical reactions that require precise temperature control and high-pressure conditions. Efficient energy management is essential for controlling costs and minimizing the environmental impact of production.
Waste Management and Environmental Compliance: The production process generates chemical by-products and waste that need to be properly managed to comply with environmental regulations. Implementing waste treatment systems and emission control technologies is essential for ensuring sustainable and environmentally responsible operations.
By optimizing the procurement of raw materials, technology, and energy, manufacturers can streamline the amiodarone production process and reduce overall costs.
Amiodarone (Cordarone): An Overview
Amiodarone is a highly effective antiarrhythmic medication used to treat irregular heartbeats. It is classified as a class III antiarrhythmic agent, but it exhibits properties of multiple antiarrhythmic classes, making it versatile in treating a range of cardiac arrhythmias. Its efficacy in treating ventricular tachycardia and fibrillation has made it a critical drug in both emergency and long-term management of cardiac conditions.
Key characteristics of amiodarone (Cordarone) include:
Mechanism of Action: Amiodarone works by blocking potassium channels, which prolongs the cardiac action potential and stabilizes the electrical conduction in the heart. It also inhibits sodium and calcium channels and has noncompetitive beta-blocking effects, which makes it effective in controlling various types of arrhythmias.
Therapeutic Applications: Amiodarone is widely used in the treatment of ventricular arrhythmias, atrial fibrillation, and supraventricular tachycardia. It is often administered in hospital settings for acute arrhythmia management and is prescribed for long-term use to prevent recurrence of arrhythmias.
Pharmacokinetics: Amiodarone has a long half-life, which allows it to remain active in the body for an extended period. However, it also accumulates in various tissues, which can lead to side effects such as pulmonary toxicity, liver dysfunction, and thyroid abnormalities. Due to these risks, patients on amiodarone require regular monitoring.
Given its life-saving role in cardiac care, amiodarone continues to be an essential drug in both emergency medicine and chronic cardiac management.
Market Drivers
The market for amiodarone is driven by several factors related to the growing prevalence of cardiovascular diseases and the increased demand for effective antiarrhythmic therapies. Key market drivers include:
Rising Prevalence of Cardiovascular Diseases: Cardiovascular diseases, including arrhythmias, are among the leading causes of death globally. As the global population ages, the incidence of heart-related conditions is expected to rise, driving demand for antiarrhythmic medications like amiodarone.
Increased Use in Emergency Care: Amiodarone is commonly used in emergency medical settings, particularly in the treatment of ventricular fibrillation and tachycardia, where immediate intervention is critical. Its inclusion in advanced cardiac life support (ACLS) guidelines further supports its use in emergency medicine.
Long-Term Treatment of Arrhythmias: Amiodarone is not only used for acute arrhythmia management but is also prescribed for long-term treatment to prevent recurrence. Its efficacy in managing both atrial and ventricular arrhythmias makes it a preferred choice for chronic arrhythmia management.
Growing Healthcare Access in Emerging Markets: As healthcare infrastructure improves in emerging markets, access to advanced cardiovascular treatments, including antiarrhythmic medications, is expanding. This growth in healthcare access is expected to increase the demand for amiodarone in regions where it was previously underutilized.
These market drivers indicate sustained demand for amiodarone, particularly as the global burden of cardiovascular diseases continues to grow.
Raw Materials Requirements
The production of amiodarone requires several raw materials, including chemical intermediates and reagents. The quality and availability of these materials directly impact the efficiency of the production process and the quality of the final product. Key raw materials include:
Iodinated Intermediates: Iodine is a critical component of amiodarone’s molecular structure. Iodinated intermediates are synthesized or sourced from chemical suppliers and play a central role in the production of amiodarone. Ensuring a high-quality supply of these intermediates is essential for maintaining product efficacy.
Organic Solvents: Various organic solvents, including acetone, dichloromethane, and methanol, are used during the synthesis and purification stages of amiodarone production. These solvents facilitate the chemical reactions and aid in the separation and crystallization of the final product.
Catalysts and Reagents: Catalysts are used to accelerate chemical reactions, while reagents such as acids, bases, and oxidizing agents are required for specific synthesis steps. The choice of catalysts and reagents can impact the reaction efficiency and yield, making them critical components in the production process.
Water: High-purity water is required for the preparation of solutions, rinsing, and crystallization processes. Water quality is crucial to ensure the purity of the final product and prevent contamination.
Maintaining a consistent supply of high-quality raw materials is essential for ensuring the efficiency and cost-effectiveness of the amiodarone production process.
Costs and Key Process Information
The cost of producing amiodarone is influenced by several factors, including raw material costs, energy consumption, labor, and capital investment in production facilities. Key cost components include:
Raw Material Costs: The price of iodinated intermediates, solvents, and reagents plays a significant role in determining the overall cost of production. Fluctuations in the availability and price of these materials can impact production costs, making efficient procurement strategies essential.
Energy Costs: The production of amiodarone involves several energy-intensive steps, including chemical synthesis and purification. Energy costs can vary depending on the geographical location of the production facility and the availability of efficient energy sources.
Labor and Operating Costs: Skilled labor is required to operate chemical reactors, monitor the production process, and ensure quality control. Labor costs can vary based on the level of automation in the facility and the local wage rates.
Capital Expenditures: The production of amiodarone requires specialized equipment, including reactors, filtration systems, and crystallization units. Investing in modern, automated equipment can reduce labor costs and improve production efficiency, but it requires significant upfront capital investment.
Regulatory and Compliance Costs: The production of pharmaceutical-grade amiodarone requires adherence to strict regulatory standards, including Good Manufacturing Practices (GMP). Compliance with these regulations adds to the overall cost of production but is essential for ensuring product safety and efficacy.
By carefully managing these cost factors and investing in modern technologies, companies can optimize the production process and improve profitability.
Looking for an Exhaustive and Personalized Report to Substantiate Your Business?
For businesses seeking a detailed analysis of the amiodarone production process and market dynamics, a personalized and exhaustive report can provide valuable insights to support strategic decision-making. A customized report can include:
In-Depth Cost Analysis: A detailed breakdown of production costs, including raw material procurement, energy consumption, labor, and capital investments, tailored to your specific production facility.
Market Demand Forecasts: Insights into future demand for amiodarone in key markets, including pharmaceuticals, healthcare, and emergency medicine, allowing you to plan for capacity expansions and market entry strategies.
Regulatory Compliance and Quality Control: Guidance on meeting regulatory requirements, ensuring product safety, and maintaining high standards in the production of pharmaceutical-grade amiodarone.
Supply Chain Optimization: Recommendations for sourcing high-quality raw materials, managing procurement logistics, and ensuring a reliable supply chain to meet growing demand.
Partnering with industry experts to develop a comprehensive, data-driven report can help your business navigate the complexities of the amiodarone market, enhance production efficiency, and ensure long-term business success.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: [email protected]
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA